Design Characteristics of Mesoporous Silica Nanoparticles
In addition to their biocompatibility, key advantages of MSNs are associated with their high surface area and facile surface tunability. Often described as "nanosponges," MSNs can adsorb large amounts of molecules dissolved in solution thanks to this high surface area; in fact, 5 grams of mesoporous silica nanoparticles possess the same surface area as an American football field. This highly ordered porosity can be controlled during the synthesis of MSNs.
Tunable Pore Sizes for Size-Selective Adsorption
MSNs can be made to have very narrow pore size distributions, yielding nanoparticles that will selectively adsorb molecules of a specific size. The figure below illustrates that only molecules within a specific size range (small green circles) will enter the MSN cavities, while other larger molecules (large red circles) will be excluded. This feature is useful for molecule separation by size exclusion.
Figure 2 – pore size comparison of MSNs
Tunable Surface Chemistry for Functionally-Selective Adsorption
Thanks to the versatility of the silica, it’s possible to modify the surfaces of MSNs with specific functional groups that will interact selectively with certain categories of molecules. One can imagine coating the pores with a positive surface in order to attract negatively charged molecules, or using a hydrophobic surface to attract more hydrophobic molecules. The figure below shows an MSN in which the pores are modified to allow molecules with a specific surface functionality (represented as green circles) to adsorb inside the particles, whereas others (red pentagons) are excluded. This type of selective adsorption has numerous uses in hydrophobic drug encapsulation, environmental chemical removal, and various other applications.
Figure 3 – diagram of MSN with modified pores
Variable Pore Structures for Controlled Release
The organizational structure of the pores can also be modulated (MCM-41, MCM-48, radial, cubic, wormlike, etc.), as represented in the following figure. The use of one type over the others will depend on the application, as the pore structure controls the release/leakage of molecules loaded inside the pores.
Figure 4 – different organizational structures of MSN pores
For example, MSNs with the hexagonal pore arrangement can release cargo from both ends of a specific channel whereas in the cubic arrangement, molecules can travel freely inside the intricate network and be released from any pore outlet. In the radial pore arrangement, the molecule can only access a single pore and thus must exit through the pore it entered, making this particular system less prone to premature leaking (in the case of drug encapsulation).
Applications of Mesoporous Silica Nanoparticles
In general, we can look to the unique structural properties of MSNs to justify how these materials are used. Here you’ll find a brief overview of how these properties are useful to biomedicine and catalysis.
Nanomedicine
Over the past 15 years, the myriad benefits of MSNs have majorly impacted the drug delivery field, evidenced by the rising number of scientific articles involving their use as drug carriers.2–4 Their high surface area, easy surface modification, and biocompatibility make them the material of choice for targeted delivery of active pharmaceutical ingredients for cancer therapy and other treatments.2 The high surface area of MSNs allows them to carry larger amounts of the desired drug than through conventional methods (e.g. polymers, liposomes, and vesicles), and with the size-selective capabilities discussed previously. Moreover, the easy surface modification and biocompatibility makes them useful for targeted delivery of active pharmaceutical ingredients with different chemistries such as molecules with hydrophilic and/or hydrophobic properties. The surface functionalization allows for the potential targeting of specific tissues or cells, or the increase of the enhanced permeability and retention (EPR) effect by evading the reticuloendothelial system (RES). Although mesoporous silica is generally robust, MSNs can be engineered to be degradable to facilitate controlled release of their contents.5
Catalysis
The easily adjustable surface chemistry gives MSNs a unique affinity for loading catalysts onto their high surface areas. MSNs (MCM41-type, in particular) have shown shape selective capabilities of the substrate due to the narrow pore size distribution. This has been observed in the epoxidation of bulky alkene substrates using titanocene-grafted-MSN structures.6 Grafting can be achieved through activating the surface silanols (or other surface chemistry) by displacing the ligands of the catalyst species and attaching to the inorganic core. Inorganic catalysts can also be integrated into the structure of the MSNs to increase catalytic efficiency, for instance titanium can be integrated into a MCM41-structured zeolite for oxidation.7 The cartoon below illustrates the use of MSNs for catalysis, where the analyte (green circles) penetrates the pores of the particles where the catalysis reaction happens, and a new product is then formed and released (purple squares).
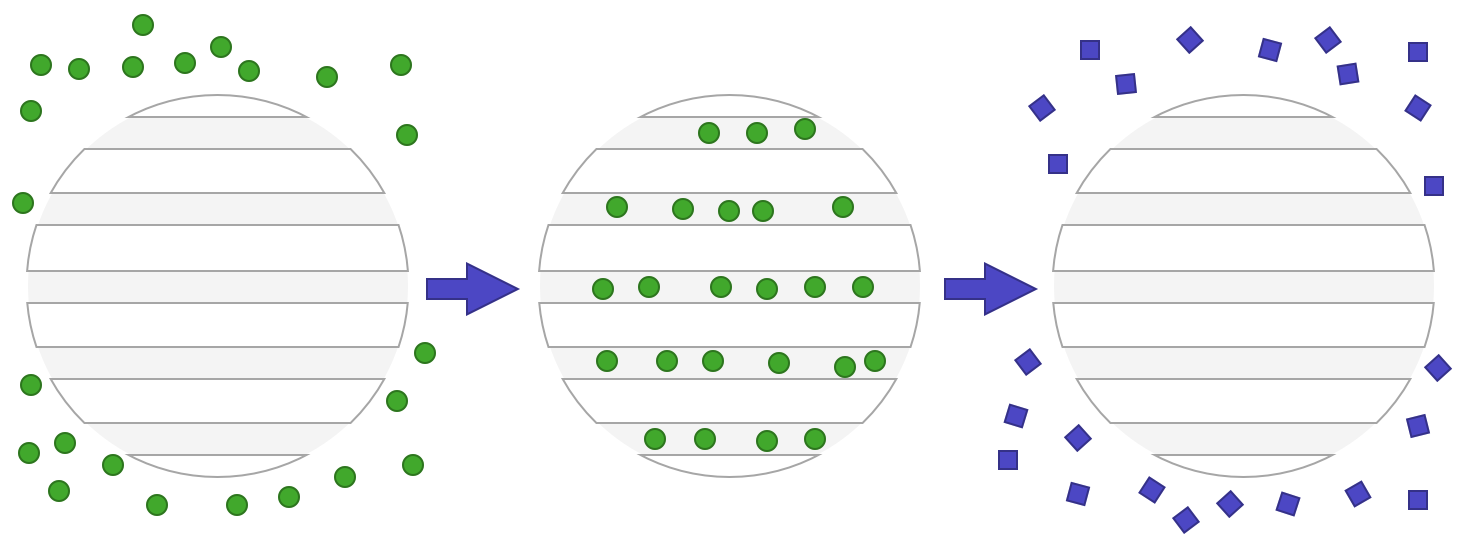
Figure 5 – illustration of MSNs used in catalysis
Furthermore, surface functionalized MSNs have been shown to exhibit catalytic capability on their own using general acid/base chemistry without the use of transition metals. As an example, it has been demonstrated using bi-functionalized MSNs with both an acid and base group to perform carbonyl activation for Aldol, Henry, and cyanosilylation reactions. Examination of the reaction rates based on the varying ratios of acid/base functionalization suggest the MSNs take part in the reaction mechanism.8
A key benefit of MSNs is the ease of centrifugation instead of requiring chemical separation to isolate product, which decreases the resources needed and makes the process greener. The mechanical separation won’t damage the catalysts or mesoporous framework so the catalytic material can be regenerated or recycled easily.
Synthesis & Purification of Mesoporous Silica Nanoparticles
Compared to the synthesis of regular silica nanoparticles that generally follow the Stöber process, MSNs are synthesized in a water-based solution in the presence of a base catalyst and a pore forming agent more widely known as a surfactant. Surfactants are molecules that present the particularity to have a hydrophobic tail (alkyl chain) and a hydrophilic head (charged group, such as a quaternary amine for example). As these surfactants are added to a water-based solution, they will coordinate to form micelles with increasing concentration in order to stabilize the hydrophobic tails as shown below.
Figure 6 – structure of surfactants and their arrangement into micelles
During the early stage of the hydrolysis and condensation of the silica precursor, oligomeric forms of silica appear. Like the final material, these oligomers possess silanol groups (Si-OH) that are deprotonated under the basic conditions of the reaction, forming negatively charged oligomers that can in turn condense on the surface of the positively charged micelles. As the reaction proceeds, the oligomers grow larger around the micelles ultimately forming a hybrid organic/inorganic silica network templated by surfactant molecules. After the reaction, the removal of the template will create the porosity that defines mesoporous silica nanoparticles.
Figure 7 – chemical structure of CTAB
Purification: The Importance of Removing CTAB for Biological Applications
After synthesis, CTAB must be removed for practical use of MSNs. This is important for three main reasons:
Pore accessibility
The presence of the surfactant inside the pores will reduce the capability of the particles to encapsulate any active pharmaceutical ingredient (API) by decreasing the pore volume.
Cytotoxicity
Most of the common surfactants used to synthesize mesoporous silica nanoparticles are harmful to the body. The widely used CTAB molecule is able to interact with the phospholipids constituting the cell membrane which could lead to cell death at high concentrations. As a result it is crucial to ensure total removal of these pore forming agents prior to biomedical application, especially injections.
Pore surface modification
Removal of the surfactant molecules from the pores will make them accessible to further modification such as amination, thiolation, or hydrophobization. Doing so makes it possible to have the same functionality throughout the entire silica surface (inside the pores and outside of the particles), or to have two distinct types of functionality which will bring different capabilities to each spatial region. An example would be the loading of a negatively charged molecule inside the pores (after amine modification of the inner surface) while having the outside of the particle functionalized with thiol or any other functional group for targeted drug delivery.
