4 Products
Nanoparticles for Diagnostics
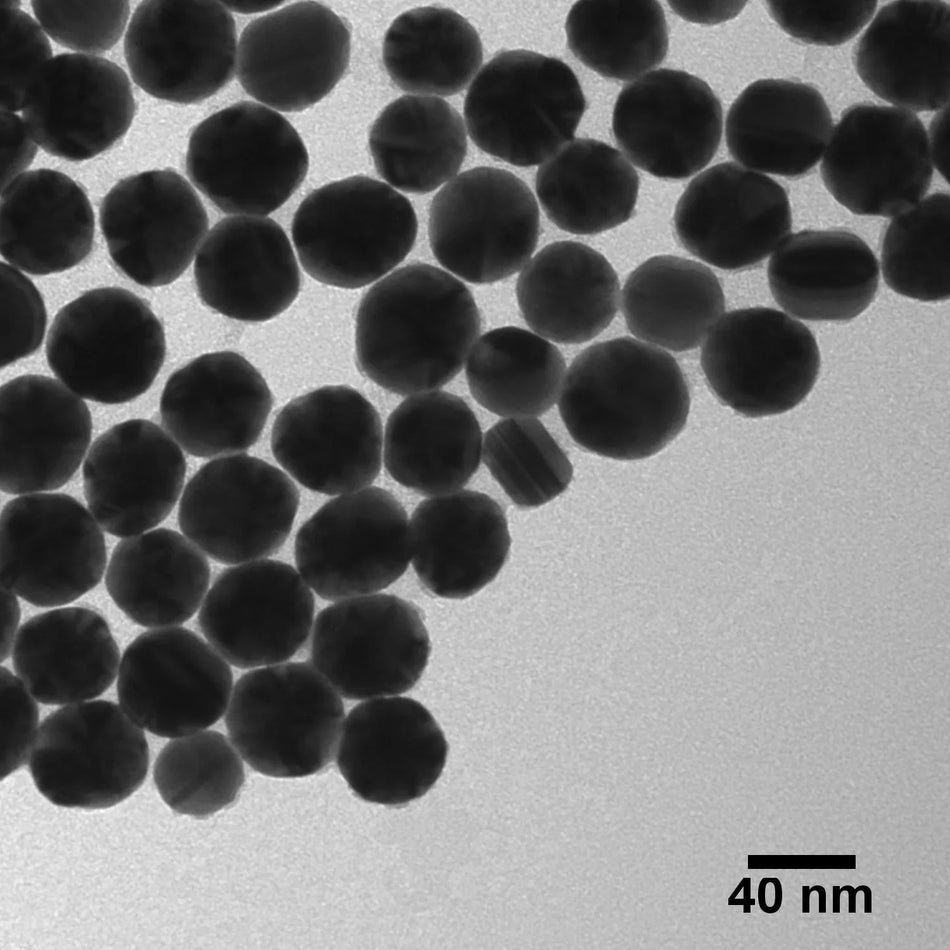
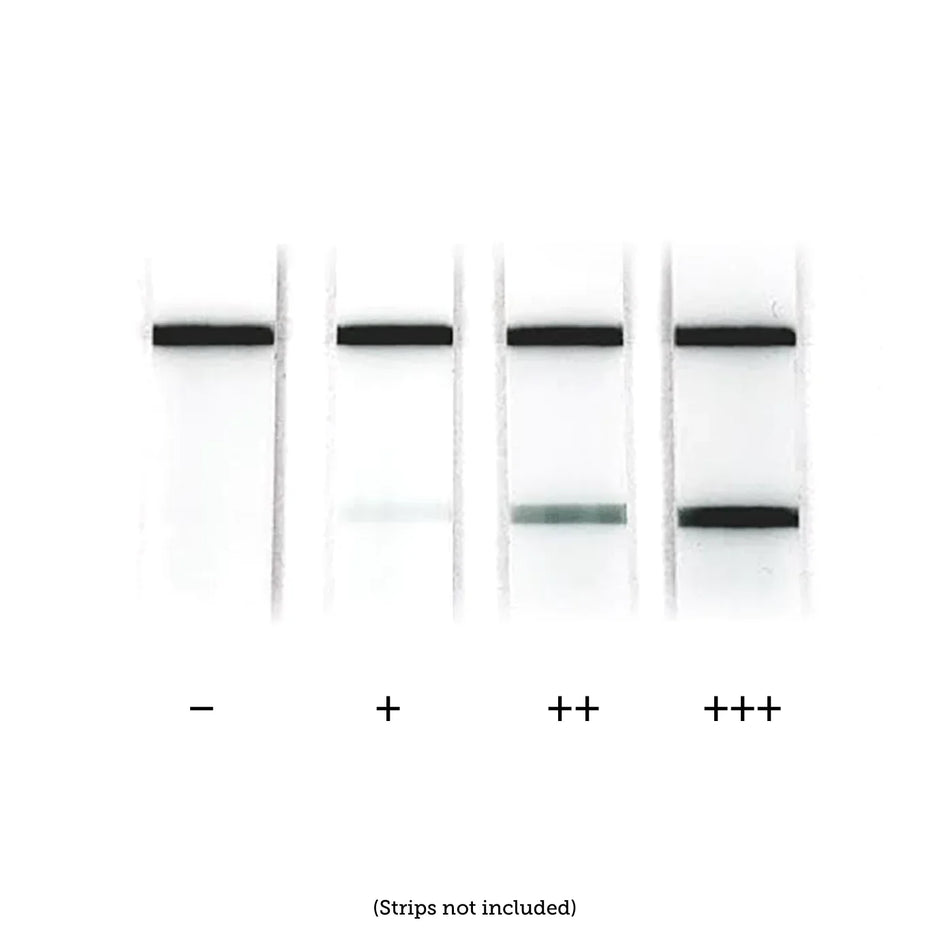
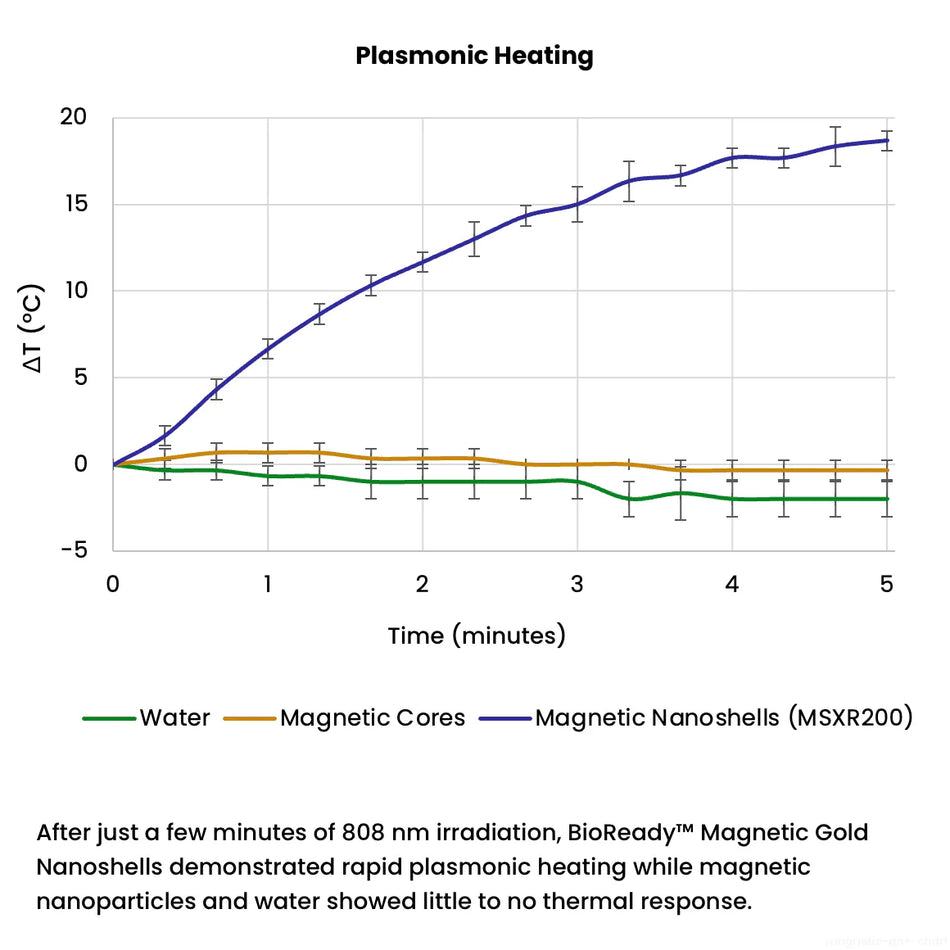
Nanoparticles play an important role in a wide range of diagnostic devices and testing methods to increase performance (e.g. sensitivity, speed) or to enable a detection method that would not be possible without nanotechnology. Plasmonic nanoparticles are extremely strong absorbers and scatters of light and are used in lateral flow diagnostics, surface enhanced spectroscopy, cell labeling, and color changing sensors. Nanoparticles with electrochemical, magnetic, or fluorescent properties response are also used in many different diagnostic applications.