General Information
Our silica nanoparticles are produced via the condensation of silanes to form nanoparticles that consist of an amorphous network of silicon and oxygen via the Stöber method. The particles are monodispersed with narrow size distributions. The refractive index of silica is estimated at 1.43. The density of silica nanoparticles is approximately 2 g/cm3 and depends on the degree of condensation. Colloidal silica is amorphous or non-crystalline, meaning that the atoms do not have long-range order, similar to the atomic structure of bulk glass. As prepared, they are readily suspended in polar solvents such as water and ethanol. By binding different silanes to the nanoparticle surfaces, they can be rendered hydrophobic for suspension into non-polar matrices.
Size Control
Spherical silica nanoparticles can be produced at large scale with sizes ranging from tens of nanometers to micrometers in diameter. By changing reaction conditions such as the concentration of reactants, the catalyst used, and the reaction temperature, the sizes of the particles can be easily tuned. For example, increasing the concentration of water and ammonia results in larger nanoparticles, whereas increasing the concentration of tetraethyl orthosilicate (TEOS) yields smaller particles.
The TEM images below illustrate that careful control over the growth conditions produces uniform particles of various sizes with low coefficients of variation (CV). In fact, silica particles are known for their low CVs and have even been suggested for use as size standards to aid in calibration of materials characterization tools.1
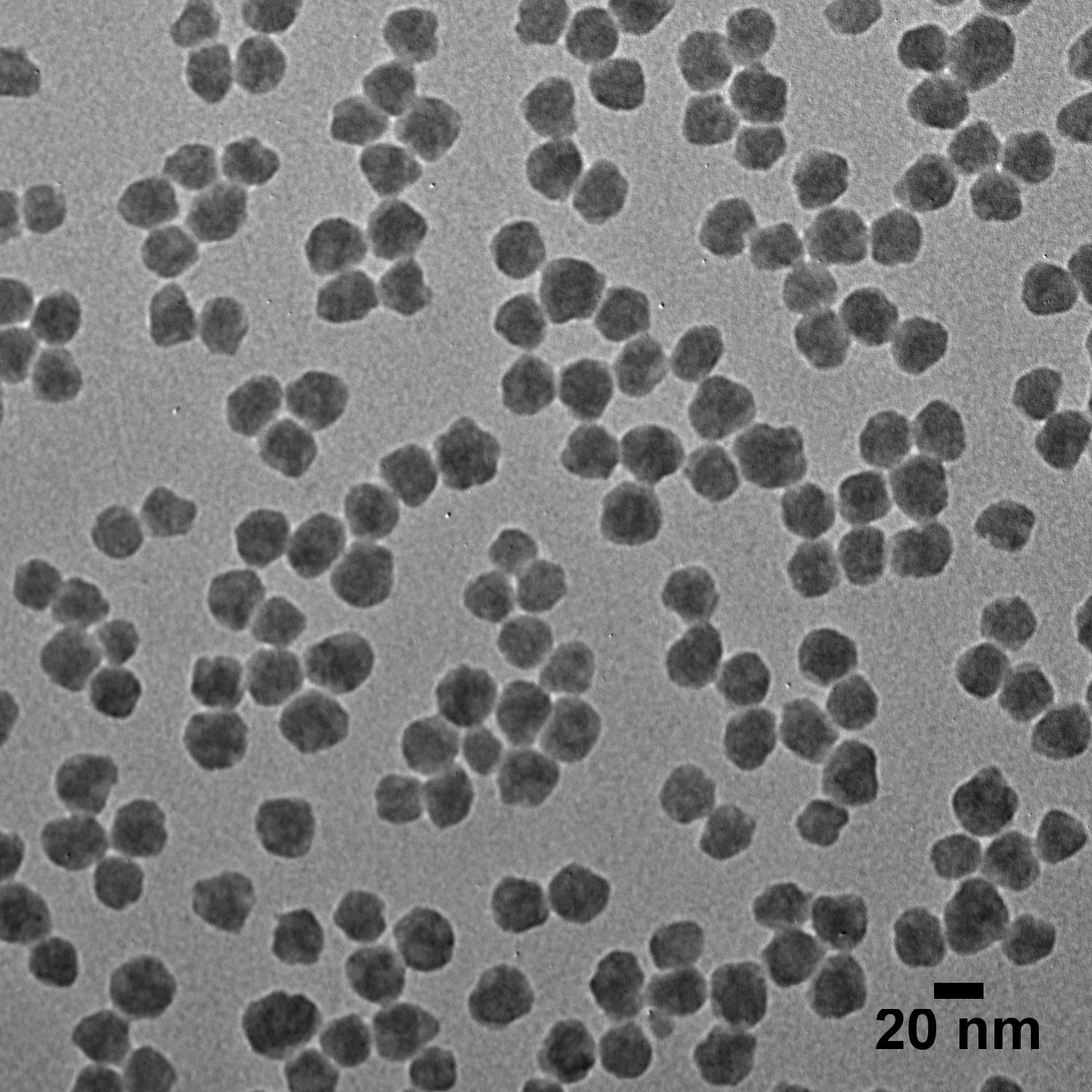
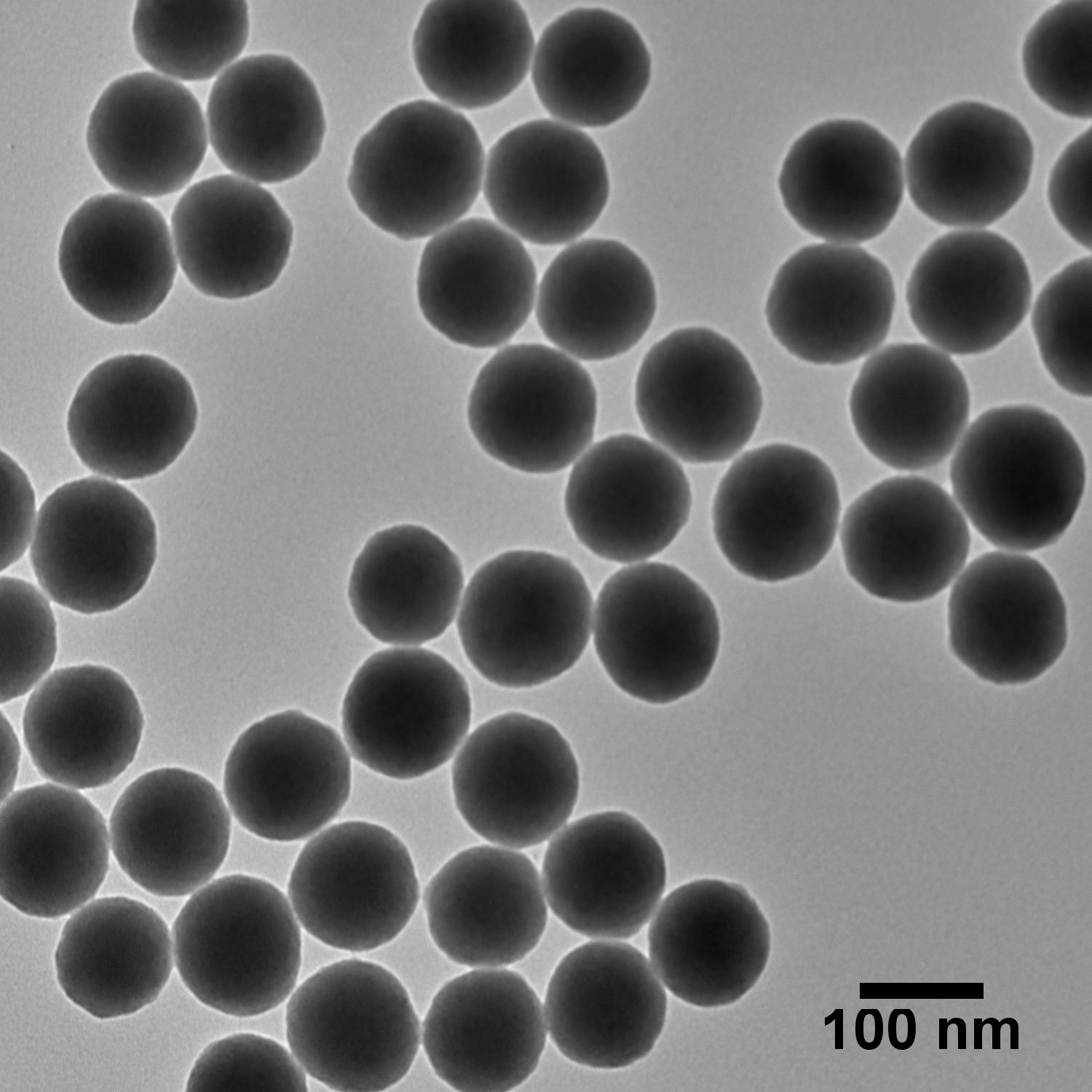
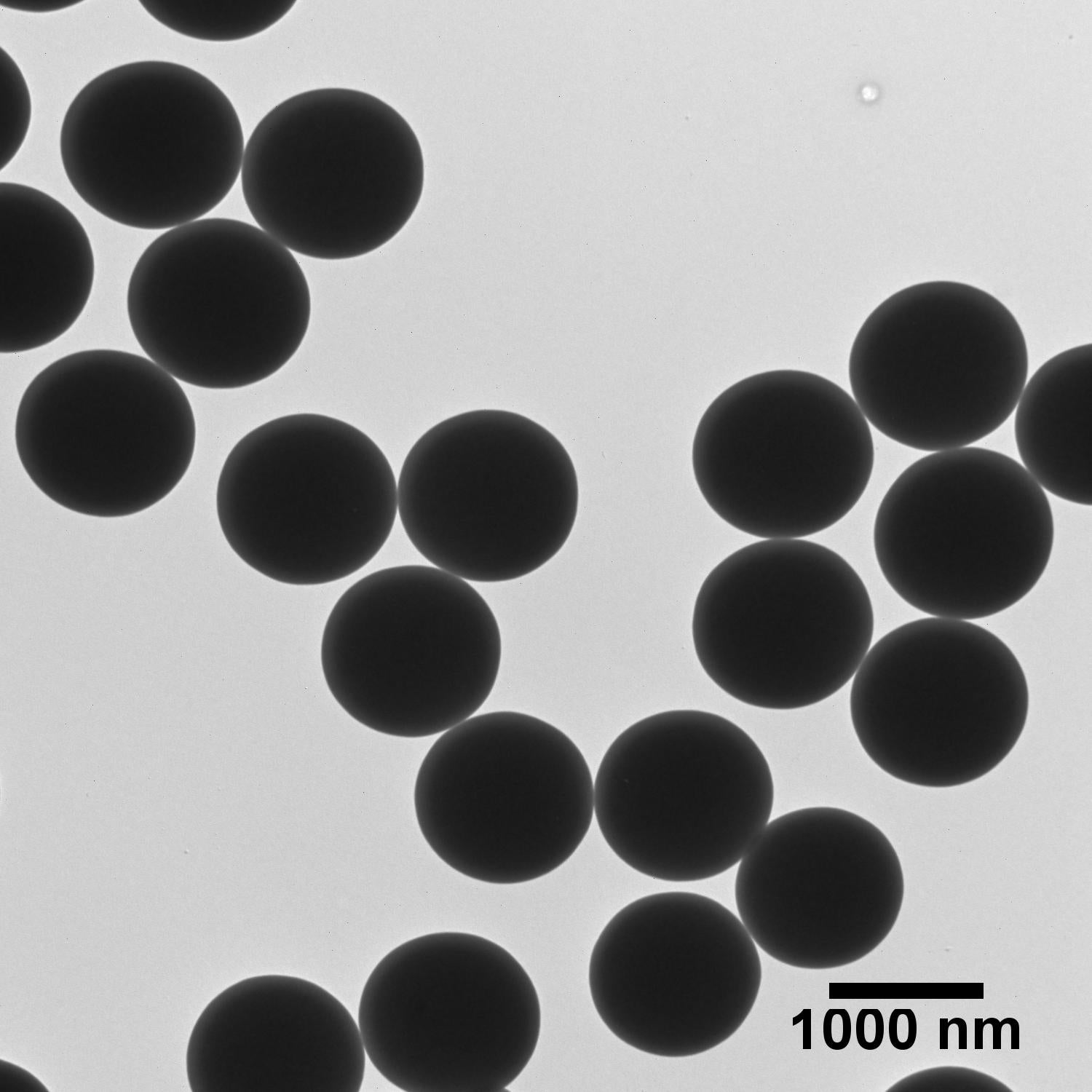
Silica Solvent Compatibility
Dispersible in: ethanol, water, isopropyl alcohol (IPA), dimethyl sulfoxide (DMSO), ethylene glycol, dimethyl formamide (DMF)
Not readily dispersible in: non-polar organic solvents
Interested in silica compatibility that is not listed here? Read on to learn about silica surface chemistry and our custom synthesis capabilities.
Silica Surface Functionalization
Silica is known for its rich surface chemistry that can be easily functionalized with hundreds of silanes. Common silica surfaces include amino-, mercapto-, carboxy-, and aldehyde-terminated depending on the application of interest. At nanoComposix, we have extensive experience fabricating particles with custom sizes and surface chemistries, conjugating particles to a variety of proteins and antibodies, and incorporating fluorophores, quantum dots, or dyes within colloidal silica particles. For more information please visit our Custom Synthesis page or contact us.
NanoXact Silica Nanospheres are available with a negatively-charged bare (non-functionalized) silica surface and a positively charged amine-terminated surface. Non-functionalized (hydroxyl-terminated) silica is ideal for physadsorption of small molecules or for applications requiring high particle stability. Aminated silica is used for covalent binding to molecules such as dyes, proteins and antibodies.
Hydroxyl-Terminated Silica
Bare silica nanoparticles have very good colloidal stability in water and alcohols. The silica surface is very versatile and can be readily modified to attach specific functional groups. This can include creating hydrophobic, or fluorophilic, surfaces. The nanoporous structure of silica allows moderate molecular weight molecules, such as fluorophores or drug molecules, to be loaded into the silica shell.
The hydroxyl groups give silica colloids a large negative zeta potential at neutral and basic pH. Zeta potential measurements of 80 nm-diameter silica colloids as a function of pH show that the isoelectric point of silica nanoparticles is close to pH 2. This indicates that at pH > 2, the surfaces will be negatively charged and functionalized with Si-O– groups.

For help reading and interpreting the data above, check out our Zeta Potential Tutorials. To learn more about this type of surface chemistry, try out our Silica Surface module.
Amine-Terminated Silica
Amine-functionalized silica is useful for binding studies, conjugation with carboxyl-containing molecules through EDAC coupling, or binding to dyes and molecules with isothiocyanate (ITC) or amine-reactive esters. The amines at the colloid surface can be protonated at acidic pH to yield particles with a large positive zeta potential. Measurement of zeta potential versus pH for 120 nm amine-terminated silica particles indicates an isoelectric point near pH 7.5.

Based on the amount of reagent used during the surface functionalization step and the surface area available for the ligand to bind, we calculate a maximum of ~2.5 amine groups/nm2 at the particle surface. This is consistent with literature reports,2 which estimate approximately two amine groups/nm2. Depending on orientation, packing density, and other factors, only a portion of the amines may be accessible for conjugation. Further, in some cases there are also amine groups that are incorporated into the silica network below the particle surface, and which contribute to the zeta potential of the particle and can be detected using different characterization methods. Because they are embedded within the silica shell, however, these amines are not accessible for conjugation.
NanoComposix’s amine-functionalized silica is provided in ethanol in order to preserve the integrity of the amine functional groups on the surface, as silica has much higher solubility in water and under some solution conditions the silica network will partially dissolve, leading to a loss of some functional groups. While ethanol provides good material stability, it is also slightly basic with a pH of approximately ~7.0–7.5, which happens to be very close to the isoelectric point of the amine-functionalized silica. This can cause certain sizes of amine-functionalized silica to flocculate and fall out of solution because of the low surface charge present under these solution conditions. This phenomenon is separate from large diameter nanoparticles settling over time due to gravity. This can be reversed by dispersing the silica in a low pH acidified buffer, such as acetate at pH 5. Sizes > 50 nm can be centrifuged down and redispersed in a low pH buffer. Contact us for details.
Silica Nanoparticle Concentration
Different commercial vendors report concentration using various methods. When researching which nanoparticles to purchase and use it is critical to be able to compare formulations from different suppliers. There are a number of different ways that the concentrations are reported. Here we provide tables converting our silica nanoparticle formulations into other commonly reported concentration units.
| Size (nm) |
Mass Concentration (mg/mL) |
Particle Concentration (particles/mL) |
SiO2 Mass Percent (%) |
Optical Density at λ350 (cm-1) |
|---|---|---|---|---|
| 20 | 10 | 1.1 × 1015 | 1.0 | 0.049 |
| 50 | 10 | 6.9 × 1013 | 1.0 | 0.79 |
| 80 | 10 | 1.7 × 1013 | 1.0 | 2.94 |
| 100 | 10 | 8.7 × 1012 | 1.0 | 4.24 |
| 120 | 10 | 5.0 × 1012 | 1.0 | 4.87 |
| 140 | 10 | 3.2 × 1012 | 1.0 | 6.74 |
| 160 | 10 | 2.1 × 1012 | 1.0 | 8.73 |
| 180 | 10 | 1.5 × 1012 | 1.0 | 10.55 |
| 200 | 10 | 1.1 × 1012 | 1.0 | 10.87 |
Silica Solubility
Silica has low but non-negligible solubility in water. At pH values above 8 and below 3, the solubility of silica increases rapidly. In dilute concentration, silica can dissolve from particle surfaces until equilibrium is reached. This is especially noticeable for silica shells on metal nanoparticles. At low concentrations, silica shells can be removed completely in just a few hours. The rate of dissolution can be modified by exposure to aluminum chloride, heating, or reduced temperature. One the other hand, the slow silica dissolution can be advantageous, enabling dried silica to be deagglomerated by resuspending in a slightly basic solution.
Silica Porosity
The extent of silica nanoparticle porosity is highly tunable and can be carefully controlled during synthesis by varying the degree of condensation of hydroxide (–OH) groups through a process called olation. Porosity can also be controlled through hydrolysis and dissolution of siloxane bonds, heat treatment with sodium borohydride, and post-synthetic etching under basic conditions.3 Solid silica nanoparticles are only slightly porous relative to metal nanoparticles, whereas hollow and mesoporous silica nanoparticles have large internal pores that are often spacious enough to incorporate small molecules or even large biomolecules into the nanoparticle’s interior.

The figure above shows that each tetraethyl orthosilicate (TEOS) molecule in the silica network can couple to as many as four other silane molecules through oxygen atom bridges. However, during standard sol-gel growth and coating procedures, there are still many hydroxides present in the coating. These unbound –OH groups reduce the silica density and thus increase its porosity; more complete condensation can be driven by heating particles. This tunable porosity lends itself to ready fabrication of mesoporous silica and hollow porous silica nanostructures. Interestingly, silica can be hollowed even in juxtaposition with other materials for controlled formation of heterostructures and core-shell nanoparticles with porous silica engineered into different spatial regions of the particles.
Other Variants of Silica
Alumina Silica (Aluminosilicate)
Aluminosilicate refers to the class of materials in which aluminum atoms are substituted for some of the silicon atoms. Several aluminosilicate polymorphs exist in nature and can be made synthetically, and similar to silica, these materials are amorphous. Aluminosilicates are particularly well known for their strength at high temperatures, beyond that of other related glasses and on the order of properties typically associated with ceramics.
One very effective method for increasing the robustness of silica nanoparticles in water is to convert some portion of the incompletely condensed silica surface into aluminosilicate. This surface conversion significantly reduces the dissolution of silica and preserves its surface chemistry. Aluminosilicate can also be deposited as shells on the surfaces of different types of nanoparticle cores; for instance, magnetic nanoparticles to enhance their stability and/or facilitate their binding to DNA.
Studded Silica
Nanoparticles with varying identities can also be attached to silica surfaces with a wide variety of biomedical and technological applications in mind. For instance, silica shelled gold nanoparticles can be studded with fluorescent quantum dots (QDs) on the surface. The fluorescence from the QDs can be used to track the particles for nanomedicinal applications.
Interested in these varieties? Learn more about our Custom Synthesis capabilities.
References
- Kimoto, S.; Dick, W. D.; Hunt, B.; Szymanski, W. W.; McMurry, P. H.; Roberts, D. L.; Pui, D. Y. H. Characterization of Nanosized Silica Standards. Aerosol Sci. Tech. 2017, 51 (8), 936-945.
- Schiestel, T.; Brunner, H.; Tovar, G. E. Controlled Surface Functionalization of Silica Nanospheres by Covalent Conjugation Reactions and Preparation of High Density Streptavidin Nanoparticles. J. Nanosci. Nanotechnol. 2004, 4 (5), 504-11.
- Liu, S.; Han, M.-Y. Silica-Coated Metal Nanoparticles. Chem. Asian J. 2010, 5, 36-45.



