Gold nanoparticles absorb and scatter light with extraordinary efficiency. Their strong interaction with light occurs because the conduction electrons on the metal surface undergo a collective oscillation when they are excited by light at specific wavelengths. This oscillation is known as a surface plasmon resonance (SPR), and it causes the absorption and scattering intensities of gold nanoparticles to be much higher than identically sized non-plasmonic nanoparticles. Gold nanoparticle absorption and scattering properties can be tuned by controlling the particle size, shape, and the local refractive index near the particle surface.
The Effect of Size on Optical Properties
The optical properties of spherical gold nanoparticles are highly dependent on the nanoparticle diameter. The extinction spectra of 15 sizes of NanoXact Gold nanoparticles at identical mass concentrations (0.05 mg/mL) are displayed in the figure below. Smaller nanospheres primarily absorb light and have peaks near 520 nm, while larger spheres exhibit increased scattering and have peaks that broaden significantly and shift towards longer wavelengths (known as red-shifting). Larger spheres scatter more light both because they have larger optical cross sections, and also because their albedo (a ratio of scattering to total extinction) increases with size. Gold nanoparticles are often used as bioimaging tags in dark field microscopy techniques, where the scattering from individual nanoparticles with diameters larger than 40-50 nm can be observed.


The Effect of Local Refractive Index on Optical Properties
Gold nanoparticle optical properties also depend on the refractive index near the nanoparticle surface. As the refractive index near the nanoparticle surface increases, the nanoparticle extinction spectrum shifts to longer wavelengths (known as red-shifting). Practically, this means that the nanoparticle extinction peak location will shift to shorter wavelengths (blue-shift) if the particles are transferred from water (n=1.33) to air (n=1.00), or shift to longer wavelengths if the particles are transferred to oil (n=1.5). The figure below displays the calculated extinction spectrum of a 50 nm gold nanosphere as the local refractive index is increased. Increasing the refractive index from 1.00 to 1.60 results in an extinction peak shift of over 40 nm, moving the peak from the green to the red region of the spectrum. When embedded in high index materials, the extinction cross section is substantially increased.

Similarly, the extinction peak can be tuned by coating aqueous nanoparticles with nonconducting shells including silica (n=1.5), biomolecules (n=1.4-1.45), or aluminium oxide (n=1.58-1.68).
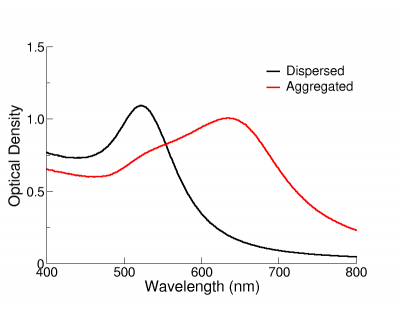
The Effect of Aggregation on Optical Properties
The optical properties of gold nanoparticles change when particles aggregate and the conduction electrons near each particle surface become delocalized and are shared amongst neighbouring particles. When this occurs, the surface plasmon resonance shifts to lower energies, causing the absorption and scattering peaks to red-shift to longer wavelengths. UV-Visible spectroscopy can be used as a simple and reliable method for monitoring the stability of nanoparticle solutions. As the particles destabilize, the original extinction peak will decrease in intensity (due to the depletion of stable nanoparticles), and often the peak will broaden or a secondary peak will form at longer wavelengths (due to the formation of aggregates). In the figure on the right, the extinction spectrum of 12 nm functionalized NanoXact gold that has carboxy (-COOH) groups on the surface is monitored as the solution pH is changed from 6.5 to 3. As the solution becomes more acidic, the carboxy group is protonated and the zeta potential is reduced resulting in destabilized nanoparticles that aggregate. The rapid change in the extinction spectrum as the pH is decreased clearly demonstrates that the nanoparticles are agglomerating. A similar signal is observed in solutions where unfunctionalized nanoparticles destabilize and aggregate. UV-Visible spectroscopy can be used as a characterization technique that provides information on whether the nanoparticle solution has destabilized over time.
 Unaggregated gold nanoparticles will have a red color in solution, as seen in the picture to the right. If the particles aggregate, the solution will appear blue/purple and can progress to a clear solution with black precipitates.
Unaggregated gold nanoparticles will have a red color in solution, as seen in the picture to the right. If the particles aggregate, the solution will appear blue/purple and can progress to a clear solution with black precipitates.





