- Introduction to drug delivery enabling properties of nanoparticles
- Nanoparticle selection guidelines
- Examples of drug delivery applications
Introduction to Drug Delivery Enabling Properties of Nanoparticles
 Drug delivery is a notoriously difficult task, with numerous variables affecting the efficacy of a given active ingredient. The integration of tailored nanoparticles into drug delivery applications has been used to address limitations of conventional drug delivery, particularly challenges associated with targeting and timed release.
Drug delivery is a notoriously difficult task, with numerous variables affecting the efficacy of a given active ingredient. The integration of tailored nanoparticles into drug delivery applications has been used to address limitations of conventional drug delivery, particularly challenges associated with targeting and timed release.
Designing a nanoparticle-enabled drug delivery system includes many intersecting considerations, including method of delivery, the target location of the drug payload, uptake kinetics, and circulation time. A key lever in this design process is the functionalization of the nanoparticle surface. The introduction of functional surface chemistry groups enables attachment of a milieu of targeting ligands that will help to guide the nanoparticle to its desired location within the body, such as organ-specific cell membrane proteins, circulating biomarkers, or other disease-specific proteins. Appropriately designed surface chemistries also enable the nanoparticle to withstand its environment prior to delivery. These designs vary dependent on the requirements of the delivery method, with different considerations for oral, topical, intravenous, or inhalation delivery routes.
 A nanomedicine may be needed to deliver a therapeutic, to inhibit or promote enzymatic activity, to upregulate or down-regulate a particular pathway, induce apoptosis, or deliver genetic material. The nanomedicine needs to not only deliver the payload to the correct location but needs to maintain the appropriate concentration (the “therapeutic window) for sufficient time to be efficacious. To accomplish this, the nanomedicine needs to circulate in the body long enough to release its targeted drug payload and maintain a therapeutic concentration before the body’s immune system clears the nanoparticle. Nanoparticle quality by design (QbD) considerations for this end application include: Will the nanomedicine need to be administered once or multiple times? What are the desired drug release kinetics? Do the nanoparticles accumulate in the body or what is the clearance pathway? The ability to tailor the nanoparticle composition, size, shape, and surface functionalization allows the nanomedicine’s release, circulation and clearance profiles to be tailored to address these questions.
A nanomedicine may be needed to deliver a therapeutic, to inhibit or promote enzymatic activity, to upregulate or down-regulate a particular pathway, induce apoptosis, or deliver genetic material. The nanomedicine needs to not only deliver the payload to the correct location but needs to maintain the appropriate concentration (the “therapeutic window) for sufficient time to be efficacious. To accomplish this, the nanomedicine needs to circulate in the body long enough to release its targeted drug payload and maintain a therapeutic concentration before the body’s immune system clears the nanoparticle. Nanoparticle quality by design (QbD) considerations for this end application include: Will the nanomedicine need to be administered once or multiple times? What are the desired drug release kinetics? Do the nanoparticles accumulate in the body or what is the clearance pathway? The ability to tailor the nanoparticle composition, size, shape, and surface functionalization allows the nanomedicine’s release, circulation and clearance profiles to be tailored to address these questions.
Along with customization options for nanoparticle based delivery systems, this format offers an additional benefit in the ability to encapsulate cytotoxic drug payloads at high loadings while masking their presence prior to targeted delivery. This format builds on recently approved antibody drug conjugates (ADCs) used as new chemotherapeutic agents, for example, in which a monoclonal antibody (mAb) is covalently attached to a drug molecule to improve the delivery of cytotoxic materials to tumors compared to traditional administration routes. Since few molecules of the drug can be attached to each antibody, however, ADCs suffer from relatively low drug loading. By using porous or hollow nanoparticles that are engineered to encapsulate the drug while targeting specific locations in the body, the highly concentrated payload and reduction in non-specific delivery may improve therapeutic efficacy and patient outcomes.
References
- Mitchell, Michael J., et al. “Engineering precision nanoparticles for Drug Delivery.” Nature Reviews Drug Discovery, vol. 20, no. 2, 2020, pp. 101–124. doi.org/10.1038/s41573-020-0090-8.
Related Material
Nanoparticle Selection Guidelines
The selection of nanoparticle type and surface chemistry for drug delivery applications depends on a variety of factors, such as delivery route, chemical properties and toxicity of the drug to be loaded, the desired drug concentration in the nanomedicine, and desired lifetime within the body.
Nanoparticle Material and Morphology
Silica Nanoparticles: Solid or porous silica (amorphous SiO2) nanoparticles can be reproducibly prepared across a range of sizes, with high uniformity, and modified via standard silane chemistry to introduce a wide variety of surface functionalization possibilities. Although silica is an inorganic material, it is a common compound in biological creatures and can be biodegraded then ultimately renally excreted. Due to this combination of biocompatibility and biodegradability, silica nanomaterials are the delivery vehicle of choice for a diverse range of active pharmaceutical ingredients (APIs). Solid silica particles have nanopores that can incorporate low molecular weight species, or the surface may be functionalized to be highly positively charged to electrostatically adsorb genetic material, for example to deliver silencing RNA to specific cells.
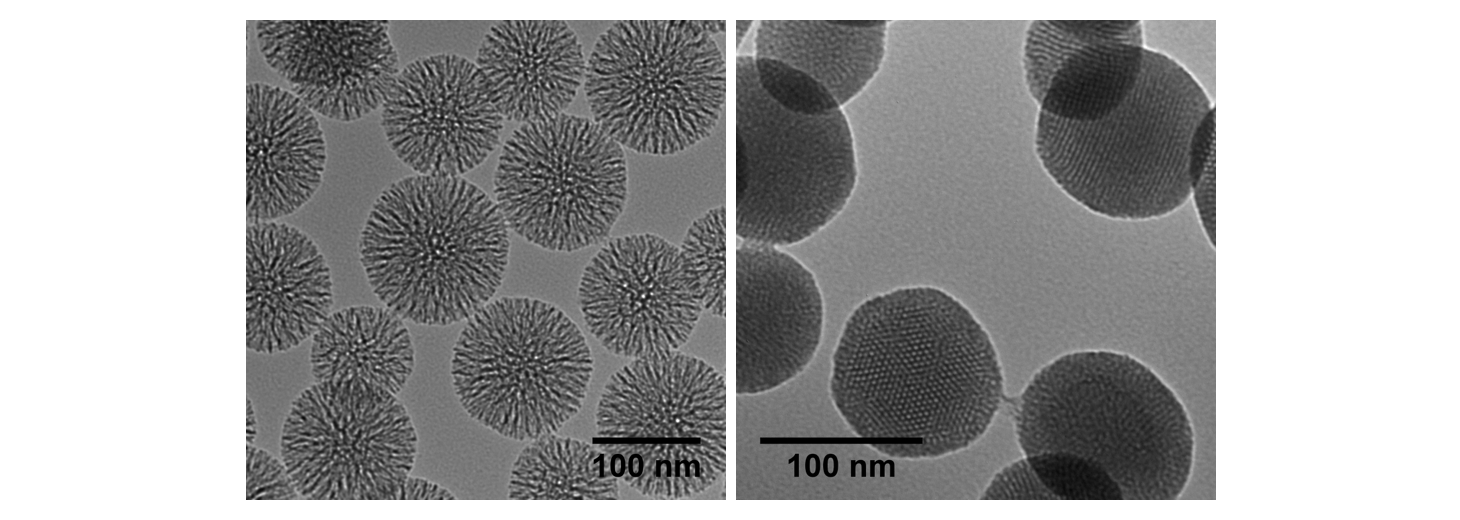
The delivery of larger drug molecules or higher loadings of payloads can be accomplished using mesoporous silica nanoparticles (MSNs), which have attracted significant attention over the last two decades primarily due to their versatility of cargo loading capability and surface functionalization. This family of biocompatible inorganic nanoparticles is able to accommodate a wide variety of APIs, from small hydrophobic drugs to large biomolecules, by simply varying the size of its pores and selective surface modification, which can often be independently tailored to introduce different functionality either inside or outside its porous matrix. High loading capability up to 30% by weight for small molecules in mesoporous silica has been demonstrated, and it is not uncommon to reach 10% by weight for messenger RNA (mRNA) in large pore particles.
Download our MSN Loading Protocol
Other types of nanoparticles may be incorporated into the core of solid and mesoporous silica nanoparticles, creating composite particles with multiple functionalities or to introduce particles that may act as tracers. For example, silica shells with tunable thickness and surface chemistry may be deposited around Au cores, and such particles may be used in pharmacokinetic, pharmacodynamic, and biodistribution studies, where the concentration and location of such particles may be determined by elemental analysis of Au, which does not occur naturally within the body.
| Application | Key Design Parameter* | Advantage | Visual Representation |
|---|---|---|---|
| ✓ Drug Delivery ✓ Catalysis ✓ Chemical Removal |
Hexagonal pore geometry | Controlled molecular adsorption & release |
 MCM-41** MCM-41** |
| Negative (silica) or positive (aminated) surface functionality options | Ability to perform specific chemical transformations |  |
|
| 100 nm particles with 3 nm pores | Accommodates most small molecules & drugs |  |
|
| Large pore radial geometry | Accommodates larger APIs |  |
*Certain design parameters require a custom design – contact us to learn more
**Radial and cubic pore geometries also available as custom materials
Metal Nanoparticles:  Gold is the most widely used metal for nanoparticle drug delivery and therapeutic applications, due to the inert chemical properties of the material and general biocompatibility. Current interest in gold nanoparticles in nanomedicine typically exploit the unique optical properties of gold, specifically the localized surface plasmon resonance (LSPR) that can be tuned from visible to near infrared (NIR) wavelengths by changing the particle size and morphology. As a material with advanced capabilities in both the therapeutics and diagnostics realms, gold-based nanoparticles can be readily utilized as theranostics agents, combining these two application areas, and have been utilized in diverse applications ranging from two-photon luminescence to photodynamic and photothermal therapeutics, to identification of cells via Surface Enhanced Raman Spectroscopy (SERS).
Gold is the most widely used metal for nanoparticle drug delivery and therapeutic applications, due to the inert chemical properties of the material and general biocompatibility. Current interest in gold nanoparticles in nanomedicine typically exploit the unique optical properties of gold, specifically the localized surface plasmon resonance (LSPR) that can be tuned from visible to near infrared (NIR) wavelengths by changing the particle size and morphology. As a material with advanced capabilities in both the therapeutics and diagnostics realms, gold-based nanoparticles can be readily utilized as theranostics agents, combining these two application areas, and have been utilized in diverse applications ranging from two-photon luminescence to photodynamic and photothermal therapeutics, to identification of cells via Surface Enhanced Raman Spectroscopy (SERS).
PLGA Nanoparticles: Over the past few decades, nanoparticles fabricated from poly(lactic-co-glycolic acid) (PLGA) have been attractive vehicles for drug delivery due to FDA approval of the polymer for therapeutic uses and the biocompatibility and biodegradability of the material. This class of copolymer can be made with different ratios of lactic acid and glycolic acid to adjust material properties and the degradation rate in the body, and the terminal end groups of the polymer can also be chosen to create PLGA terminated with carboxylic acids, esters, and amines, providing a great deal of versatility in nanoparticle design and functionalization. Depending on the application needs, the synthesis route for the PLGA particles may be tuned to yield uniform particles in sizes between 100 nm to 1 µm. The combination of these characteristics allows us to encapsulate a wide range of APIs including hydrophobic and hydrophilic small molecules, proteins, and RNA, with typical loadings up to 10 wt% of API.
Read more about our PLGA fabrication and manufacturing capabilities

Nanoparticle Surface Modification
Implementing nanoparticles for drug delivery applications requires the selection of not only the appropriate particle size, shape, and material type but also the appropriate surface chemistry and targeting ligands. At nanoComposix, we can modify the surface of many nanoparticles with specific functional groups, biocompatible polymers, and biomolecules (proteins, antibodies, oligonucleotides). The table below summarizes some of the most common surface modifications employed by nanoComposix for drug delivery applications.
| Modification | Description |
|---|---|
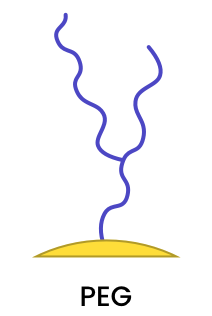 |
Polyethylene glycol (PEG) ligands offer a high degree of biocompatibility, can improve stability in high salt environments, and reduce non-specific interactions. PEG ligands can be covalently bound to the metal surface to produce stable coatings. In addition, certain bi-functional PEGs can be employed to introduce other surface functionalization. |
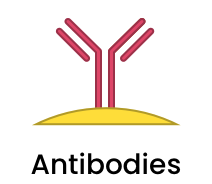 |
Antibodies (Ab), including fragments and VHH single domain antibodies can be conjugated onto a variety of nanoparticles using standard EDC/NHS chemistry or via a host of bioorthogonal click chemistry methods, depending on the particle type. |
 |
Proteins are able to be conjugated onto a nanoparticle surface using the same type of conjugation chemistry used for Ab. If more than one protein per nanoparticle needs to be conjugated onto a nanoparticle, this can be accomplished using bioorthogonal click chemistry. |
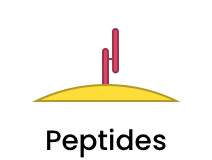 |
Sometimes only a short fragment is needed to direct the nanomedicine to the correct target, and can be accomplished by conjugating specific peptide sequences to the nanoparticle surface. |
 |
Oligonucleotides (oligos) can be covalently attached or encapsulated. |
 |
Various fluorophores can be covalently attached or may be encapsulated within the nanoparticle. |
Triggered Drug Release Using Gold Nanoparticles
Functionalized gold nanoparticles can release payload in appropriate location through irradiation by near-infrared (NIR) light leading to plasmonic heat generation. With the ability to be decorated with a variety of compounds including doxorubicin, hyaluronic acid, folate, polymers (PEG, poly(N-isopropylacrylamide), proteins (ovalbumin, biotin) and DNA and RNA (siRNA and shRNA), gold nanoparticles provide a versatile platform for delivering therapeutic cargos through the thermal stimulation of heat-responsive coatings.
- Tao, Yu, et al. “Light: A magical tool for controlled drug delivery.” Advanced Functional Materials, vol. 30, no. 49, 2020. doi.org/10.1002/adfm.202005029
- Moorcroft, Samuel C., et al. “Nanoparticle-loaded hydrogel for the light-activated release and photothermal enhancement of antimicrobial peptides.” ACS Applied Materials & Interfaces, vol. 12, no. 22, 21 2020, pp. 24544–24554. doi.org/10.1021/acsami.9b22587
- Vines, Jeremy B., et al. “Gold nanoparticles for photothermal cancer therapy.” Frontiers in Chemistry, vol. 7, 2019. /doi.org/10.3389/fchem.2019.00167
- Gao, Qinyue, et al. “Gold nanoparticles in cancer theranostics.” Frontiers in Bioengineering and Biotechnology, vol. 9, 2021. hdoi.org/10.3389/fbioe.2021.647905
- Kennedy, Laura C., et al. “A New Era for cancer treatment: Gold‐nanoparticle‐mediated thermal therapies.” Small, vol. 7, no. 2, 2010, pp. 169–183. doi.org/10.1002/smll.201000134
- Liu, Ji, et al. “Gold nanorods coated with mesoporous silica shell as drug delivery system for remote near infrared light‐activated release and potential phototherapy.” Small, vol. 11, no. 19, 2015, pp. 2323–2332. doi.org/10.1002/smll.201402145
Silica and Mesoporous Silica Nanoparticles – A Multifunctional Platform
Silica nanoparticles can be engineered to specific sizes, and are able to tolerate low pH and withstand enzymatic digestion. As such, both solid silica and mesoporous silica nanoparticles have been used for several oral drug delivery applications, with bioavailability of APIs increasing by 50-350%. Because of their high surface area, mesoporous silica nanoparticles (MSNs) can encapsulate a wide variety of APIs at high loading levels. Both total particle size and the pore size of mesoporous silica nanoparticles can be controlled, and the surface and interior of the particle may be independently functionalized in some cases. For example, genetic materials may be adsorbed on the outer surface of positively charges MSNs – obtained through a simple one-step surface modification – while smaller drug molecules can be effectively trapped inside the pores. For sensitive materials such as messenger RNA, it is possible to increase the size of the cavity to accommodate their larger size and protect them from the environment. In addition, straightforward surface modification approaches can be used to functionalize them with pH, temperature or enzyme-responsive capping molecules to further direct delivery of their cargo under prescribed or triggered conditions. The versatility of silica allows the creation of composite core-shell materials, incorporating nanoparticles of gold, iron oxide or silver as the cores surrounded by a uniform silica shell, which allows for either release of cargo upon irradiation, magnetic activation, or synergistic drug-metal cation effects.
References
- Janjua, Taskeen I., et al. “Clinical Translation of silica nanoparticles” Nature Reviews, Materials, 2021, 6, 1072-1074. doi.org/10.1038/s41578-021-00385-x
- Vallet-Regi, M.; Rámila, A.; del Real, R. P.; Pérez-Pariente, J. “A New Property of MCM-41: Drug Delivery System.” Chem. Mater. 2001, 13 (2), 308–311. doi.org/10.1021/cm0011559
- Croissant, J. G.; Butler, K. S.; Zink, J. I.; Brinker, C. J. Synthetic Amorphous Silica Nanoparticles: Toxicity, Biomedical and Environmental Implications. Nature Reviews Materials 2020, 5 (12), 886–909. doi.org/10.1038/s41578-020-0230-0
- Buchman, Y. K.; Lellouche, E.; Zigdon, S.; Bechor, M.; Michaeli, S.; Lellouche, J.-P. Silica Nanoparticles and Polyethyleneimine (PEI)-Mediated Functionalization: A New Method of PEI Covalent Attachment for siRNA Delivery Applications. Bioconjugate Chem. 2013, 24 (12), 2076–2087. doi.org/10.1021/bc4004316
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators. Acc. Chem. Res. 2020, 53 (8), 1545–1556. doi.org/10.1021/acs.accounts.0c00280
- Liberman, A., Mendez, N., Trogler, W. C., & Kummel, A. C. “Synthesis and surface functionalization of silica nanoparticles for nanomedicine,” Surface Science Reports, 2014, 69(2-3), 132-158. doi.org/10.1016/j.surfrep.2014.07.001
- Popat, Amirali, et al. “Programmable drug release using bioresponsive mesoporous silica nanoparticles for site-specific oral drug delivery.” Chem Comm. 2014, 50, 5547-5550. doi.org/10.1039/C4CC00620H
- Saint-Cricq, P.; Wang, J.; Sugawara-Narutaki, A.; Shimojima, A.; Okubo, T. “A New Synthesis of Well-Dispersed, Core–Shell Ag@SiO2 Mesoporous Nanoparticles Using Amino Acids and Sugars.” J. Mater. Chem. B 2013, 1 (19), 2451–2454. doi.org/10.1039/C3TB20210K
- Saint-Cricq, P.; Deshayes, S.; Zink, J. I.; Kasko, A. M. “Magnetic Field Activated Drug Delivery Using Thermodegradable Azo-Functionalised PEG-Coated Core–Shell Mesoporous Silica Nanoparticles.” Nanoscale 2015, 7 (31), 13168–13172. doi.org/10.1039/C5NR03777H
- Zhao Q, Sun X, Wu B, et al. “Construction of homologous cancer cell membrane camouflage in a nano-drug delivery system for the treatment of lymphoma.” J Nanobiotechnol 19, 8, 2021. doi.org/10.1186/s12951-020-00738-8
- Zhao, Q, Wu B, Shang Y, et al. “Development of a nano-drug delivery system based on mesoporous silica and its anti lymphoma activity.” Applied Nanoscience, 2020. doi.org/10.1007/s13204-020-01465-0
PLGA – Facile Drug Delivery System Platform
By adjusting the degradation rate of PLGA, researchers can control how quickly drugs are released, allowing for sustained release and potentially more efficient drug delivery systems (DDS). Furthermore, PLGA nanoparticles can be modified at the molecular level to target specific tissues, improving drug delivery and potentially reducing side effects in healthy cells. This targeted approach, along with the ability to monitor drug delivery through imaging, has revolutionized in-vivo techniques for diseases like cancer, cardiovascular diseases, and central nervous system disorders.
References
- Danhier, Frederic, et al. "PLGA-based nanoparticles: An overview of biomedical applications." Journal of Controlled Release, vol. 161, no. 2, 2012, pp. 505-522. doi.org/10.1016/j.jconrel.2012.01.043
- Ghitman, Jana, et al. "Review of Hybrid PLGA Nanoparticles: Future of Smart Drug Delivery and Theranostics Medicine." Materials & Design, vol. 193, 2020, p. 108805. doi.org/10.1016/j.matdes.2020.108805
- Mir, Maria, et al. "Recent Applications of PLGA Based Nanostructures in Drug Delivery." Colloids and Surfaces B: Biointerfaces, 2020. dx.doi.org/10.1016/j.colsurfb.2017.07.038