Introduction
Gold colloid – sometimes referred to as “colloidal gold” – is a suspension consisting of sub-micron gold nanoparticles suspended within a solvent, most often water. Gold nanoparticles have unique optical, electronic, and thermal properties and are being incorporated in a wide variety of technologies including microscopy, electronics, diagnostics, and therapeutics.
There are many important criteria that must be considered when selecting gold colloid for us in any of these technologies. In all cases, a desirable material will have the following characteristics.
- Unaggregated and monodisperse with narrow size distributions
- Dispersible in the desired solvent
- Available at high concentrations
- Posesses a well defined surface (sometimes referred to as a capping agent)
- Long shelf life and stable in a variety of conditions
- Highly purified with low concentrations of residual chemicals from the manufacturing process.
Gold nanoparticles can be supplied in either a dried powder or a colloidal nanoparticle formulation, and the most appropriate format depends upon the end application. During the past 6 years, nanoComposix has developed proprietary technologies to manufacture high quality powdered and solution-phase colloidal gold nanoparticles, and these materials have been sold to thousands of customers for applications ranging from solar cells to medical diagnostics. The following sections provide information about gold nanoparticles, summarize our experiences handling different types of gold nanomaterials, and discuss potential challenges that customers may face when integrating gold nanoparticles into their applications and experiments. Explore our gold colloid portfolio for purchase options.
Gold Nanoparticle Formulation Details
When selecting a gold nanoparticle supplier, it’s important to take into account a number of criteria including size distribution, aggregation state, concentration, and stability. These are discussed in more detail in the following section.
Synthesis of Gold Colloid
At nanoComposix, gold nanoparticles are fabricated in solution by reducing chloroauric acid (HAuCl4) in the presence of a variety of other chemical reagents that can be used to tune gold nanoparticle size, shape, and surface capping agent. The first step in the reaction is the precipitation of gold ions into very small particles known as “seed” nanoparticles. These seed nanoparticles then act as nucleation sites as the remaining gold ions undergo reduction, and the nanoparticles grow until the gold ions or reducing agent is depleted. By optimizing the reaction kinetics and stoichiometric ratios of gold ions and other reagents, monodisperse gold nanoparticles ranging from 5 – 250 nm can be fabricated. Two of the most famous descriptions a of these types of reactions are journal articles written by Frens1 and Turkevich2, and are excellent resources for those interested in learning more about these types of reactions. Other non-solution based methods of producing gold nanoparticles such as exploding wire or laser ablation are also utilized, however these methods produce polydisperse material with broad size distributions.
Gold Colloid Size Distribution
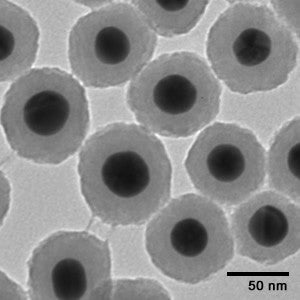
Colloidal gold nanoparticles can be produced with a very narrow size distribution. NanoComposix colloidal gold nanoparticles can achieve CVs (a measure of the standard deviation/diameter) of 8-15% for all of its colloidal formulations within the 5 nm – 100 nm size range. Figure 1 shows transmission electron microscopy (TEM) images of nanoComposix gold nanoparticles across a range of sizes. The narrow size distributions are preferable for almost all applications, as gold nanoparticles have size dependent diffusion rates, optical properties, conductivities, and electron densities.

Gold Colloid Aggregation State
The aggregation state of gold nanoparticles is considered to be the most important difference between material in colloidal and powdered formats. Studies indicate that it is the size of the aggregate, and not the size of the primary particle, that will dictate the material's properties, making it highly desirable for materials to be unaggregated. Dried commercially available nanopowders surveyed by nanoComposix were all aggregated to cluster sizes of 100 - 500 nm regardless of the primary diameter of the nanoparticle. One of the main advantages of colloidal formulations is that the gold nanoparticle solutions can be produced in an unaggregated format with hydrodynamic sizes comparable to the primary size.
Recently, nanoComposix has developed a proprietary technology to process monodisperse colloidal gold nanoparticles into a dried powder that easily redisperses without agglomeration. This gold nanoparticle formulation is ideal for applications that require dried powders, ultrahigh concentration gold solutions, or require gold nanoparticles to be mixed with other reagents prior to use.

Gold Colloid Concentration
For many applications it is desirable to have a high concentration solution available from which to dilute material in other solvents or buffers. Typically, the concentration of gold nanoparticles is limited by the synthesis method and ranges from 0.01 - 0.2 mg of gold per mL of solution. At nanoComposix, we have developed proprietary processing techniques to increase the concentration of as-produced colloidal material by a factor of 1000 or more without aggregating the material. Additionally, this same process allows for the "washing" of the colloid with buffer solutions to remove all residual reactants resulting in a high purity solution that contains only nanoparticles and surface capping agents. The same techniques can be used to expose the colloidal material to other molecules that displace the existing molecules on the surface, resulting in a new surface state. Further purification results in the removal of excess molecules to yield a new surface with no residuals present in the final formulation.
Gold Colloid Stability
There are several important factors that can influence the stability of colloidal gold nanoparticles. Among the most important factors are the particle sizes, concentration, capping agent, and local environment. Different capping agents provide differing levels of stability to salt, light, heat, pH, and solvents other than water. To read more about our available capping agents, please refer to our Knowledge Base.
Gold Colloid Batch Consistency
Colloidal gold synthesis is highly sensitive to the reaction conditions and must be prepared in batches where mixing rates and concentrations are highly controlled. To ensure the precision and accuracy of our products, NanoComposix monitors batch to batch consistency as part of its quality assurance program. Batches are processed and sold only after meeting specifications that include the size distribution, optical properties, hydrodynamic diameter, zeta potential, and solution pH of the nanoparticles. Specification sheets are provided with each material providing details on the extensive characterization.
Gold Colloid Purification and Functionalization
Gold nanoparticles can be purified from soluble impurities by extensively washing the nanoparticles with clean buffer solutions. The surface of the nanoparticles can be functionalized by adding more tightly binding capping reagents to the solution. Once a strong capping agent or ligand is adsorbed on the surface of the particle, it is extremely difficult to displace with a weaker capping agent. For instance, gold nanoaprticles synthesized in the presence of tannic acid have tannic acid that cannot be displaced from the surface by the more weakly binding citrate, thus the tannic acid remains bound the surface. However, if tannic acid coated nanoparticles are exposed to a more tightly binding ligand such as PVP, the tannic can be displaced and the nanoparticles become stabilized with the more tightly binding ligand.
Applications
Gold nanoparticles are incorporated into numerous technologies and applications. Some of the most common gold nanoparticle applications are discussed below.
Diagnostics
Gold nanoparticles are readily conjugated to antibodies, peptides, synthetic oligonucleotides, and other proteins due to the affinity of sulfhydryl (-SH) groups for the gold surface3-5. Gold-biomolecule conjugates have been widely incorporated into diagnostic applications, where their bright red color is used in home and point-of-care test such as the home pregnancy tests.
A recent study demonstrates application of nanoComposix gold nanoparticles in agglutination assays.6 NanoXact Citrate Gold Nanospheres were coated with Red Blood Cell Membranes (RBCM) to capture and crosslink with fibrinogen molecules in plasma, causing hemagglutination of the gold nanospheres that enabled detection of fibrinogen by spectrophotometry. Fibrinogen is an important biomarker with links to sepsis and cardiovascular diseases (CVD) and the technique used in this study showed a wide dynamic range of detection of fibrinogen from 0.01–10 mg/mL.
Therapeutics
Gold nanoparticles are being investigated as agents for photothermal- and microwave-based therapeutics7. Typically, this involves functionalizing the nanoparticle surface with an antibody or antibody fragment that specifically targets tumor cells. After reaching the tumor, the nanoparticles are illuminated with infrared or microwave radiation, both of which pass through skin and tissue, causing the nanoparticles to heat up, destroying the tumor cells.
Electron Microscopy
Gold nanoparticles labeled with antibodies that provide specific targeting are used to stain tissues and cells for subsequent imaging using a transmission electron microscope. Gold makes an excellent contrast agent for TEM imaging, as has a much larger electron density than biomolecules. Multiple sizes of gold nanoparticles, each labeled with a different antibody, can be used to carry out multiparameter experiments.
Conclusions
Nanomaterial production has many challenges, whether the final product is in powdered or colloidal form. This is particularly true when designing a material that will be used in many different applications. For this reason, nanoComposix provides a wide range of gold colloids with precisely engineered sizes, shapes, and surfaces.
References
- Frens, G. "Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions" Nature (London), Phys. Sci. 1973, 241, 20-22.
- Turkevich, J.; Stevenson, P. C.; Hillier, J. "A study of the nucleation and growth processes in the synthesis of colloidal gold" Discuss. Faraday. Soc. 1951, 11, 55-75.
- Mirkin, C.; Letsinger, R. L.; Mucic, R. C.; Storhoff, J. J. “A DNA-based method for rationally assembling nanoparticles into macroscopic materials” Nature 1996, 382, 607-609.
- Wang, Z.; Levy, R.; Fernig, D.G.; Brust, M. “The Peptide Route to Multifunctional Gold Nanoparticles” Bioconjugate Chemistry 2005, 16, 497-500.
- Johne, B.; Hansen, K.; Mork, E.; Holtlund, J. “Colloidal gold conjugated monoclonal antibodies, studied in the BIAcore biosensor and in the Nycocard immunoassay format” Journal of Immunological Methods 1995, 1, 167-174.
- Kim, I.; Lee, D.; Lee, S. W.; Lee, J. H.; Lee, G.; Yoon, D. S. "Coagulation-Inspired Direct Fibrinogen Assay Using Plasmonic Nanoparticles Functionalized with Red Blood Cell Membranes" ACS Nano 2021, Just Accepted.
- Huang, X.; Jain, P. K.; El-Sayed, I. H.; El-Sayed, M. A. “Plasmonic photothermal therapy (PPTT) using gold nanoparticles” Lasers in Medical Science 2008, 23, 217-228.




