Table of contents:
- Selecting Nanoparticles for Studies
- Understanding the Effects of Size
- Understanding the Effects of Shape
- Understanding the Effects of Surface
- Understanding the Effects of Dissolution Rate
- Residual Reactants
- Standards for Nanotoxicity Testing
Selecting Nanoparticles for Toxicology studies
Innovations in nanotechnology are generating a broad array of nanoparticles that are already incorporated into a wide variety of consumer products. Due to their rapid commercialization and corresponding exposure potential, there is concern over nanoparticle safety. Within the nanotoxicology community, there has been considerable debate about which nanomaterials are most relevant for determining hazard and risk.
A major challenge in predicting the potential toxicity of nanoparticles is their complexity. The toxicity of each nanomaterial is dependent not only on the primary characteristics of the particles (e.g. core chemistry, size, shape, crystallinity, surface and aggregation state), but also on secondary characteristics which rely on the nanoparticle interaction with the target biological systems (e.g. protein corona, dissolution rate, biodistribution). Discerning which properties are primary drivers of toxicity is complicated by the fact that the majority of commercially available nanomaterials are heterogeneous, unpurified, and are accompanied by little information concerning their manufacturing process. Toxicological profiles performed with these materials are difficult to interpret since the complexity of the starting material makes correlating physicochemical properties with the response of the endpoint unclear. Experiments performed with sets of precisely manufactured and well characterized nanomaterials with only a single modified property (e.g., core chemistry, size, shape, or surface) provide insight into the biological response of the varied property. However, for most nanomaterials of interest, such sets of materials are not available commercially or are time consuming and difficult for nanotoxicology researchers to produce and characterize in their own laboratories during their experiments.
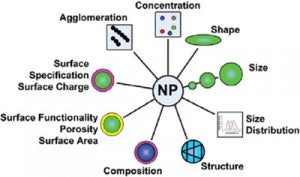
At nanoComposix, we have worked with leading researchers throughout the nanotoxicology community to develop nanoparticle formulations for toxicology research. We supply 3 of the 5 OECD silver standards and have over 200 highly characterized nanoparticle variants available for nanotoxicology studies. Over 70 different universities, research institutions, and government agencies are utilizing nanoComposix's materials for toxicological evaluation. Initial results show clear correlations between the physicochemical properties of the materials and their toxicity profiles.1,2,3
Two classes of nanoparticles are useful for conducting nanotoxicity tests:
- Precisely engineered nanoparticles where the important physical and chemical properties are tightly controlled
- Nanoparticles relevant for release that are produced in large quantities and have a high likelihood of being released into the environment.
Both of these material systems are important and are described below:
PRECISELY ENGINEERED NANOPARTICLES
One of the goals of nanotoxicology research is to link physicochemical properties to toxicity. In order to accomplish this, precisely manufactured nanoparticles must be used to probe each of the chemical and physical properties of a nanoparticle.

Examples of precisely engineered nanoparticles are BioPure Silver and Gold NPs. These particles are typically purchased for in vitro and in vivo work where each physicochemical property is tightly controlled and monitored. The BioPure line consists of nanoparticles with diameters ranging from 10 nm–100 nm with a narrow size distribution (<12% CV). Additionally, each formulation is washed of residual precursors which may impart toxicity not related to the unique physicochemical properties of the NPs. The washing and concentration of NPs without inducing aggregation is a core technology at NCX. This technology also allows for NCX to deliver nanoparticles at higher concentrations than typically available. For example, colloidal silver is typically produced at a concentration of 0.02 mg/ml. The BioPure stock is available at a 50x concentration (1 mg/ml), which is important for researchers conducting range finding and acute exposure experiments. NCX is able to concentrate materials beyond that in the BioPure line on a custom basis. Example micrographs of BioPure silver NPs are shown below.
Understanding the Effects of Size
Size is a critical parameter for designing nanotoxicology experiments. However, what is not often emphasized is the dynamic nature of particle size due to agglomeration. Primary size is defined to be the dimension of an individual nanoparticles in a suspension. The primary size is important for calculating surface area per unit mass, dissolution rates, melting point, and for defining the smallest dimension for diffusion, uptake, and biodistribution studies. When nanoparticles aggregate, they form clusters of 10's, 100's, or even 1000's of nanoparticles that have a much larger effective size than the individual nanoparticles. The hydrodynamic diameter of the aggregates can be measured using a dynamic light scattering instrument or centrifugal particle sizer and yields valuable information on the aggregation state at different time points in the experiment. For important parameters such as settling rate and biodistribution, it is the hydrodynamic size not the primary size that is the critical dimension.
To design experiments that probe the effects of size we suggest,
- If possible, use nanoparticle suspensions that are initially unagglomerated
- Select particles with a surface that is consistent across the range of sizes that you are interested
- Understand the aggregation rate upon exposure to fluids similar to those used in your experiments. For example, before introducing your nanoparticles into a cell culture experiment, determine how the particles behave in the cell culture medium alone. For many nanoparticles there is a shift in the optical properties of the particles when they agglomerate and the color of the solution can be used as an indicator for particle stability.
- If possible, obtain the same particle size with different surfaces. For example, many of our particles are available in tannic/citrate/PVP surfaces. For nanoparticles with easily displaced stabilizing agents such as citrate, other molecules can be exchanged onto the surface simply by exposing the particles to a high concentration of the new stabilant.
- Adjust your protocols to accommodate for the rate of agglomeration. For some systems, agglomeration occurs instantly, for others it may take days. Timing becomes critical to maintain reproducibility since a low level of agglomerated particles may behave completely differently than particles that have agglomerated into large clusters.
Understanding the Effects of Shape
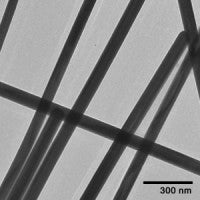
Shape is another parameter that is of interest to the nanotoxicology community. For example, it is well known that long fibers have increased toxicity in part to the difficulty of clearance. In other cases, shaped nanoparticles have well defined crystal structures that preferentially absorb different proteins in solution.
A number of silver and gold nanoparticle shape variants are available including silver nanowires and silver nanoplates. Like many of our other particles, the surface of the particles can be exchanged for different stabilants or encapsulated with a silica shell. Images of different shaped particles and their corresponding links are provided below.
Understanding the Effects of Surface
The stability, uptake, and protein corona of nanoparticles in toxicity assays are dominated by surface effects. Different surface molecules determine if the particles are positively or negatively charged, hydrophobic or hydrophillic, lipophobic or lipophillic, and the extent to which they will interact with other molecules or trigger an immune response. A core technology at NCX is the ability to functionalize or cap the surface of engineered nanoparticles with a variety of polymers, biomolecules, shells, and other surface functional groups. Examples of common surface materials used at nanoComposix are PEG, PVP, citrate, silica, phosphate, tannic acid, and biomolecules such as antibodies and DNA.

Citrate

Tannic Acid

PVP
Identifying and quantifying the surface state of a nanoparticle is important for understanding how the nanoparticle surface changes during an experiment. Once a nanoparticle is exposed to a biological system, significant changes can take place on the surface which can be an important determinant in the ultimate toxicity of the nanoparticles. Tools utilized at nanoComposix include measuring changes to the surface charge and isoelectric point of a nanoparticle, using Matrix-Assisted Laser Desorption Ionization Mass Spectrometer (MALDI-MS), Fourier Transform Infrared Spectroscopy (FTIR), and Raman spectroscopy. Protein identification is performed by dissassociating the protein from the nanoparticle surface and analyzed via LC MS/MS.
Understanding the Effects of Dissolution Rate
The dissolution of nanoparticles into their constituent ions and ion-complexes has been shown to be an important component of the toxicity of some nanomaterials. Silver ions are more toxic than silver nanoparticles on a per mass loading basis and direct correlations of the silver ion concentration of nanoparticle suspensions and toxicity have been made. One model for nanoparticle toxicity is that the nanoparticles provide a high surface area release source for toxic metal ions. Studies have demonstrated that the release rate from nanoparticles is correlated to the surface area per unit mass with larger surface areas (i.e. smaller particles) leading to higher ion release rates. For particles such as silver, this is a major factor in why increased toxicity is measured at smaller primary sizes.
The ion release rate from nanoparticles depends on the particle's size, shape, crystallinity, and surface coating. The release rate is also modulated by the ion concentration in solution and storage conditions such as temperature, exposure to oxygen/sulfur and exposure to light. Ion concentration is measured by separating the nanoparticles from the suspending medium using ultracentrifugation, chromatography, or filtration/dialysis. Once separated, the ion concentration can be measured with ICP-MS. Please contact us if you would like more information on how to measure nanoparticle dissolution rate.
Residual Reactants
Depending on the nanomaterial manufacturing method, there can be residual reactants present in the nanoparticle suspension. When testing a nanomaterial for toxicity it is important to differentiate between the toxic effects of the nanoparticles and the toxic effects of residual reactants. Controls for residual reactants can be run by spinning the nanoparticle sample in an ultracentrifuge, ensuring that all solids are compacted into a pellet, and removing the supernatant. Running the supernantant as a control will test for residual reactant effects.
The BioPure line we produce is subjected to a rigorous washing and purification process to remove excess residual synthesis precurosors that are used in the wet synthesis process. The residuals are reduced to <5 pg/ml. However, the silver nanoparticles are a continuous source of silver ions which have a known toxicity. Even with all residual reactants removed from the system, supernatants can still be toxic due to ion release from the silver nanoparticle surface.
Standards for Nanotoxicity Testing
Various organizations are identifying engineered nanomaterials for comprehensive testing:
 The OECD has established a Working Pary on Manufactured Nanomaterials (WPMN) that developed a List of Manufactued Nanomaterials and Endpoints for Countries and Stakeholders to investigate. NanoComposix is currently supplying nanoparticle standards for this effort.
The OECD has established a Working Pary on Manufactured Nanomaterials (WPMN) that developed a List of Manufactued Nanomaterials and Endpoints for Countries and Stakeholders to investigate. NanoComposix is currently supplying nanoparticle standards for this effort.
References
- Monteiro-Riviere, N.A., S.J. Oldenburg, and A.O. Inman, Interactions of aluminum nanoparticles with human epidermal keratinocytes. Journal of Applied Toxicology, 2010. 30(3): p. 276-285. Return
- Samberg, M.E., S.J. Oldenburg, and N.A. Monteiro-Riviere, Evaluation of Silver Nanoparticle Toxicity in Skin in Vivo and Keratinocytes in Vitro. Environmental Health Perspectives, 2009. 118(3): p. 407-413. Return
- Lankveld, D., Oomen, A.G., Krystek, P., Neigh, A., Troost-De Jong, A., Noorlander, C.W., Van Eijkeren, J.C., Geertsma, R.E., De Jong W.H. , The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials, 2010. 31(32): p. 8350-8361. Return




