nanoComposix has a multidisciplinary team of scientists with backgrounds in chemistry, physics, engineering, and earth sciences. We have worked with hundreds of different clients to develop nanomaterials, composites, and formulations with tailored optical, physical, and bio-functional properties.
Our expertise in the fabrication and scale-up of nanomaterials for use in products that require ISO 13485:2016* and cGMP compliant quality systems make us uniquely suited to the challenges of diagnostic applications.

Talk to one of our team members today to find out how we can help accelerate the development and commercialization of your diagnostic application.
Our services include:
- Feasibility Synthesis & Characterization
- Custom Particle Development & Optimization
- Scale-up, Process Development, & Validation
- Tailored cGMP compliant manufacturing
Looking to boost your diagnostic applications with nanoparticle technology?
Our NanoFab QuickStart package offers rapid feasibility assessments for a wide range of applications such as in-vitro imaging, antigen detection, or bioimaging. Receive conjugate or particle samples to assess the viability of your platform and accelerate your innovations.
Click a heading below to learn more about our capabilities and how they apply to diagnostic applications.
nanoComposix has deep expertise in fabricating uniform and stable metal nanoparticles with controlled size and shape in order to take advantage of the unique plasmonic and optical properties of these nanomaterials. By carefully engineering nanoparticle size, shape, and composition, the optical response can be tuned from the ultraviolet, through the visible, to the near-infrared regions of the electromagnetic spectrum. These optical properties make plasmonic nanoparticles ideal reporter probes for a wide variety of diagnostic technologies, including photonic biosensors, microscopy and imaging, colorimetric assays, and lateral flow assays. For more information on the science behind these optical properties visit our nanoComposix University site .
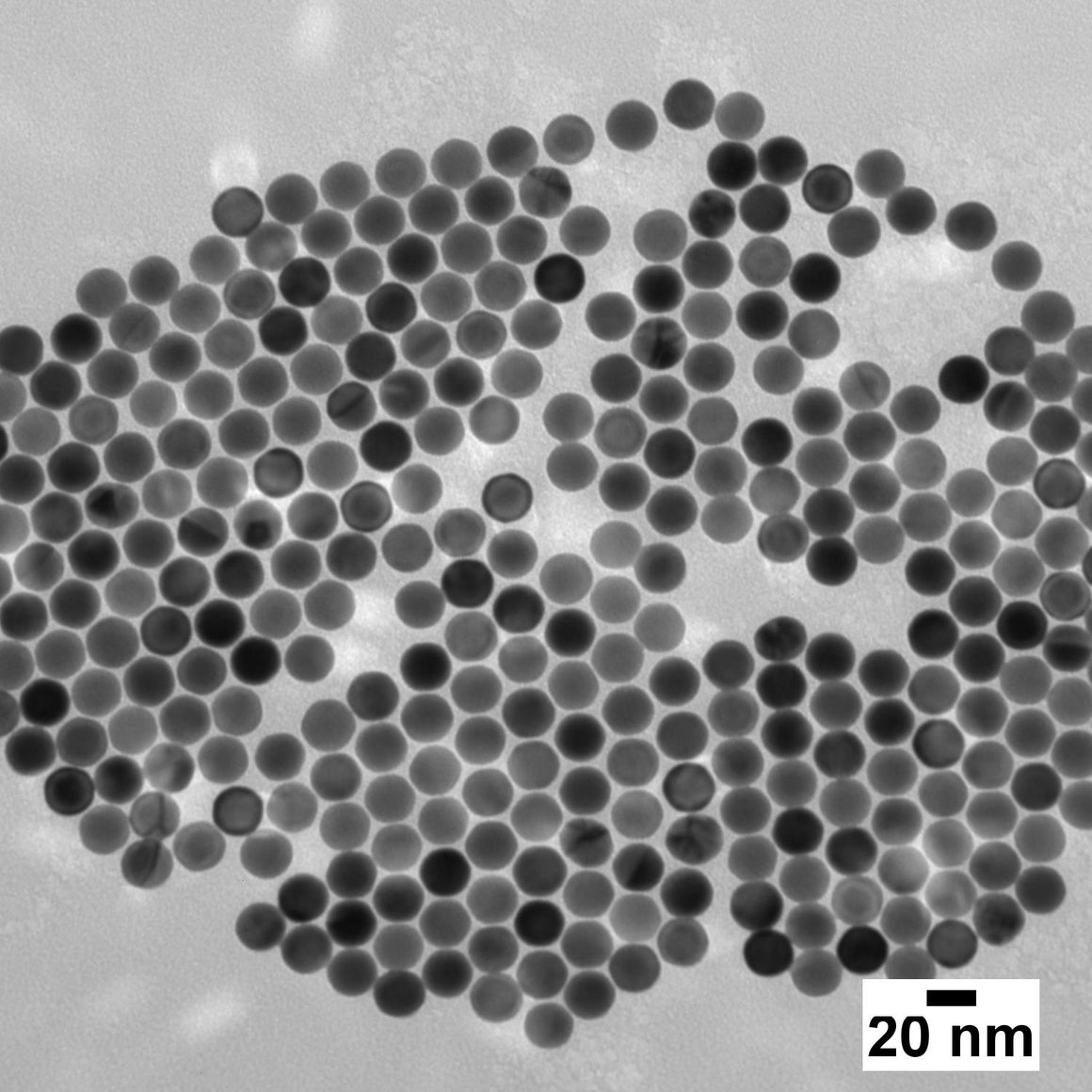


Transmission Electron Microscope (TEM) images of Ultra-Uniform gold nanospheres (top), silica-shelled gold nanospheres (middle), and gold nanorods (bottom) made by nanoComposix.
Selected Publications
- Che, C.; Xue, R.; Li, N.; Gupta, P.; Wang, X.; Zhao, B.; Singamaneni, S.; Nie, S.; Cunningham, B. T. Accelerated Digital Biodetection Using Magneto-Plasmonic Nanoparticle-Coupled Photonic Resonator Absorption Microscopy. ACS Nano 2022, 16 (2), 2345–2354. doi.org/10.1021/acsnano.1c08569.
- Waitkus, J.; Chang, Y.; Liu, L.; Puttaswamy, S. V.; Chung, T.; Molina Vargas, A. M.; Dollery, S. J.; O’Connell, M. R.; Cai, H.; Tobin, G. J.; Bhalla, N.; Du, K. Gold Nanoparticle Enabled Localized Surface Plasmon Resonance on Unique Gold Nanomushroom Structures for On‐Chip CRISPR‐Cas13a Sensing. Adv Materials Inter 2023, 10 (1), 2201261. doi.org/10.1002/admi.202201261.
Colorimetric Labeling and Imaging
The small size and unique optical properties of nanoparticles have found utility in imaging nanoscale features of inorganic and biological substances, including cell components, tissue structure, and molecular transport.
Plasmonic nanoparticles can be engineered to have very high scattering cross sections that allow sub-100 nm diameter particles to be easily detected with a standard dark field microscope, as seen below where simply changing the size of gold nanospheres significantly shifts the wavelength selective scattering by the particles. Multiplexed imaging is accomplished by using plasmonic nanoparticles with different colors, which can be selected by further tuning the nanoparticle material, size, and shape.

Dark field microscope images showing the scattered color from gold nanospheres of different size – 60 nm (left) and 100 nm (right). Each spot represents the scattering from a single nanoparticle. Changing the size, shape, and composition of the nanoparticles allows their optical properties to be tuned to provide a response at desired wavelengths.
Nanoparticles can also be fluorescently labelled, and this fluorescence can be enhanced by using a plasmonic particle that functions as an antenna for increasing brightness. Many other nanoparticle imaging formats are also utilized including Raman labels that provide a unique fingerprint for highly multiplexed imaging applications, and magnetic nanoparticles that have high contrast for MRI scans.
Selected Publications
- Koudrina, A.; O’Brien, J.; Garcia, R.; Boisjoli, S.; Kan, P. T. M.; Tsai, E. C.; DeRosa, M. C. Assessment of Aptamer-Targeted Contrast Agents for Monitoring of Blood Clots in Computed Tomography and Fluoroscopy Imaging. Bioconjugate Chem. 2020, 31 (12), 2737–2749. doi.org/10.1021/acs.bioconjchem.0c00525.
- Marangoni, V. S.; Neumann, O.; Henderson, L.; Kaffes, C. C.; Zhang, H.; Zhang, R.; Bishnoi, S.; Ayala-Orozco, C.; Zucolotto, V.; Bankson, J. A.; Nordlander, P.; Halas, N. J. Enhancing T 1 Magnetic Resonance Imaging Contrast with Internalized Gadolinium(III) in a Multilayer Nanoparticle. Proc. Natl. Acad. Sci. U.S.A. 2017, 114 (27), 6960–6965. doi.org/10.1073/pnas.1701944114.
- Shin, H.; Oh, S.; Kang, D.; Choi, Y. Protein Quantification and Imaging by Surface‐Enhanced Raman Spectroscopy and Similarity Analysis. Adv. Sci. 2020, 7 (11), 1903638. doi.org/10.1002/advs.201903638.
Photonic Biosensors
Photonic biosensors take advantage of interactions between light and target analytes as a detection mechanism. Plasmonic nanoparticles can enhance the interactions between light and the target analyte to greatly improve the sensitivity of the measurement through surface plasmon resonance effects. With our ability to biofunctionalize and tune the optical properties of particles to match specific peak wavelength, absorption, and scattering criteria, nanoComposix is uniquely suited to design and manufacture nanoparticle reporters for photonic biosensor technologies.

Incorporation of a nanoparticle in an immunoassay-based biosensor drives increased interactions with the incident laser signal through surface plasmon resonance.
Surface Enhanced Raman Scattering (SERS)



Transmission Electron Microscope (TEM) images of gold nanostars (top), gold nanoshells (middle), and silver nanocubes nanocubes (bottom) made by nanoComposix.
Raman spectroscopy can be used to identify molecules by their unique vibrational modes. While intrinsic Raman scattering of photons from molecules is weak and requires long measurement times to obtain a Raman spectrum, Surface Enhanced Raman Scattering (SERS) from molecules near the surface of plasmonic metal nanoparticles offers the potential for intensities comparable to that of fluorescent tags . Click here to learn more about SERS. With our ability to tune surface plasmon resonance and functionalize the surface of nanoparticles, we can engineer particles to meet your SERS parameters.
Selected Publications
- Shin, H.; Oh, S.; Hong, S.; Kang, M.; Kang, D.; Ji, Y.; Choi, B. H.; Kang, K.-W.; Jeong, H.; Park, Y.; Hong, S.; Kim, H. K.; Choi, Y. Early-Stage Lung Cancer Diagnosis by Deep Learning-Based Spectroscopic Analysis of Circulating Exosomes. ACS Nano 2020, 14 (5), 5435–5444. doi.org/10.1021/acsnano.9b09119.
- Shin, H.; Oh, S.; Kang, D.; Choi, Y. Protein Quantification and Imaging by Surface‐Enhanced Raman Spectroscopy and Similarity Analysis. Adv. Sci. 2020, 7 (11), 1903638. doi.org/10.1002/advs.201903638.
- Chio, W.-I. K.; Liu, J.; Jones, T.; Perumal, J.; Dinish, U. S.; Parkin, I. P.; Olivo, M.; Lee, T.-C. SERS Multiplexing of Methylxanthine Drug Isomers via Host–Guest Size Matching and Machine Learning. J. Mater. Chem. C 2021, 9 (37), 12624–12632. doi.org/10.1039/D1TC02004H.
Organic Fluorophores
Organic fluorophores are widely used for fluorescence detection, and dye families with functional groups for biomolecule labelling are widely available with emission wavelengths across the spectrum. The ability to incorporate thousands of fluorophores into a single nanoparticle offers the ability to increase assay sensitivity, where each reporter particle has a greatly enhanced signal compared to a single fluorophore.
nanoComposix has engineered methods of covalently binding dye molecules into silica nanoparticles, or into silica shells grown around core particles. In some cases, the presence of a plasmonic metal core can greatly enhance the intensity and photostability of fluorescent molecules near the surface, an effect known as surface enhanced fluorescence (SEF) or metal enhanced fluorescence.
In addition to standard fluorescent labelling, nanoComposix has previously engineered particles for SEF applications, which use a silver core surrounded by a silica shell containing a fluorescent molecule, shown below. By tuning the optical properties of the metal core and the fluorophore location within the silica shell, these SEF nanotags can be engineered to provide bright, stable fluorescence throughout the visible and near-IR spectrum. Additionally, SEF nanotags are compatible with further functionalization for biological targeting applications or to enable solvent compatibility.

Nanotags for Surface Enhanced Fluorescence (SEF).
Selected Publications
- Kim, K.; Jo, E.-J.; Hong, D.; Oh, H.-K.; Lee, K. J.; Shin, Y.-B.; Kim, M.-G. One-Pot, Solid-Phase Immunosensing Platform Consisting of a Nanometer-Thick Au/TiO 2 Photocatalytic Film and Cy5/Capture Antibody/Gold Nanorod Conjugates. ACS Appl. Nano Mater. 2021, 4 (5), 5454–5460. doi.org/10.1021/acsanm.1c00672.
- Cruz, D. F.; Fontes, C. M.; Semeniak, D.; Huang, J.; Hucknall, A.; Chilkoti, A.; Mikkelsen, M. H. Ultrabright Fluorescence Readout of an Inkjet-Printed Immunoassay Using Plasmonic Nanogap Cavities. Nano Lett. 2020, 20 (6), 4330–4336. doi.org/10.1021/acs.nanolett.0c01051.
Quantum Dots
The use of inorganic fluorophores, such as quantum dots (semiconductor nanocrystals) offers some advantages compared to traditional organic fluorophores. Quantum dots (QDs) also have tunable emission wavelengths, but have more flexibility with excitation wavelength (introducing a larger effective Stokes shift) and are typically brighter and more robust than individual organic dyes.
Like organic fluorophores, an increase in assay sensitivity can be obtained by using reporter particles containing hundreds of quantum dots and nanoComposix has developed composite particles like those shown below, in which many quantum dots are bound to a core particle. The emission from these particles can be tuned by adjusting the optical properties of the quantum dots, are highly uniform, and have surface chemistry amenable to typical biological labelling protocols. One great advantage of QDs is the ability to design highly sensitive multiplex fluorescent-based assays utilizing a single excitation wavelength.


Transmission Electron Microscope (TEM) images of quantum dot studded silica-shelled gold nanoparticles made by nanoComposix.
Magnetic nanoparticles have garnered attention for use in biomedical applications, such as magnetic resonance imaging (MRI), magnetic particle imaging (MPI), plasmonic PCR, magnetic lateral flow assays, and isolation and/or separation of target molecules. nanoComposix has developed several magnetic nanoparticle variants that have application in biomedical and life science applications. Examples of uniform single-crystal and poly-crystal iron oxide nanoparticles are shown below.



Transmission Electron Microscope (TEM) images of iron oxide nanospheres (top) iron oxide CNC’s (middle), and silica-shelled iron oxide CNC’s (bottom) made by nanoComposix.
In addition to iron oxide nanoparticles, nanoComposix has engineered several composite nanoparticles to combine magnetic properties of iron oxide nanoparticles with additional functionalities, such as magnetic-fluorescent or magnetic-plasmonic composite particles.
Magnetic-Fluorescent Nanoparticles
nanoComposix has fabricated magnetic-fluorescent composite particles, consisting of a magnetic core, silica spacer, and fluorescent quantum dots covalently bound to the silica surface. The quantum dots are functionalized to allow further coupling of biomolecules or other molecules.

Typical stucture of a magnetic-fluorescent particle (top) and TEM image (bottom).
Iron oxide nanoparticles with a diameter of ~60 nm were fabricated, coated with uniform silica shells, and covalently bound to quantum dots for a final particle diameter of ~100 nm. The reaction conditions can be tuned to modify the size of the core and shell thickness, and quantum dots with different emission properties can easily be substituted to produce samples with tailored fluorescence.
Applications for composite magnetic-fluorescent nanoparticles include multimodal imaging via optical and magnetic resonance imaging, fluorescence and magnetic activated separation, and highly sensitive lateral flow assays.
Magnetic-Plasmonic Gold Nanoshells
Gold nanoshells typically consist of a dielectric core, a thin layer of silica and a uniformly thin outer layer of gold (figure 1) with unique and tunable optical properties. This material can be used for medical diagnostic assays and photothermal therapies. Fortis Life Sciences has fabricated magnetic-responsive versions of these materials using superparamagnetic nanoparticle cores coated with a silica spacer followed by a gold shell. The optical properties can be tuned by adjusting the overall particle size or the diameter of the gold shell. The gold surface provides a useful substrate for functionalization with biological molecules or other custom surface functionality.

Typical structure of a magnetic gold nanoshells (top) and a TEM image (bottom) of magnetic gold nanoshells made by nanoComposix.
Selected Publications
- Mohammadi, M.; Antoine, D.; Vitt, M.; Dickie, J. M.; Sultana Jyoti, S.; Wall, J. G.; Johnson, P. A.; Wawrousek, K. E. A Fast, Ultrasensitive SERS Immunoassay to Detect SARS-CoV-2 in Saliva. Analytica Chimica Acta 2022, 1229, 340290. doi.org/10.1016/j.aca.2022.340290.
- Huang, P.-J.; Marks, H. L.; Coté, G. L.; Kameoka, J. A Magneto-Fluidic Nanoparticle Trapping Platform for Surface-Enhanced Raman Spectroscopy. Biomicrofluidics 2017, 11 (3), 034116. doi.org/10.1063/1.4985071.
- Mukherjee, A.; Darlington, T.; Baldwin, R.; Holz, C.; Olson, S.; Kulkarni, P.; DeWeese, T. L.; Getzenberg, R. H.; Ivkov, R.; Lupold, S. E. Development and Screening of a Series of Antibody-Conjugated and Silica-Coated Iron Oxide Nanoparticles for Targeting the Prostate-Specific Membrane Antigen. ChemMedChem 2014, 9 (7), 1356–1360. .
Implementing nanoparticles for diagnostics requires the selection of not only the appropriate size, shape, and metal type of particle, but also the appropriate surface charge and targeting ligands for the desired diagnostic application. Our catalog of gold and silver nanoparticles have a variety of different anionic and cationic capping ligands available. In addition, we can fabricate nanomaterials with specific functional groups, polymers, biomolecules, and inorganic coatings to achieve targeting and compatibility parameters. For diagnostic applications, we commonly functionalize particles with additional detection moieties, such as fluorophores or quantum dots, and biomolecules, such as antibodies, antigens, and oligonucleotides.

Our standard product catalog features different surfaces as starting platforms for surface modification and biofunctionalization allowing for both non-covalent and covalent bioconjugation. The non-covalent strategy can involve the direct physisorption between the biomolecule of interest and the metal particles through electrostatic, hydrogen bonding, and van der Waals forces.
Another form of non-covalent bioconjugation is the direct chemisorption. A small linker molecule can be present on the nanoparticle surfaces such as thiols, disulfides, terminal carboxy, amino, or maleimide groups allowing for particle and biomolecule interaction. Chemisorption can provide higher stability and can require chemical modification of the antibody or the nanoparticles.
It is important to note non-covalent strategy can result in weak and random biomolecule attachment on the nanoparticle surface and uncontrollable number of biomolecules bound per nanoparticle. An alternative strategy is covalent bioconjugation coupling strategies. For example, this can be achieved through EDC/NHS coupling where the nanoparticle has a carboxyl group on the surface which can be activated through EDC/NHS. The amine terminated biomolecule of interest is then introduced to the activated nanoparticle. The unreacted sites can then be blocked with an appropriate molecule.
The modified surface chemistry of the nanoparticles (via non-covalent or covalent approaches) can be characterized using UV-Vis, DLS, zeta potential, FTIR, and other assays.
GMP Manufacturing & Facilities
We are experts in the fabrication and scale-up of nanomaterials for use in products that require ISO 13485:2016 and cGMP compliant quality systems. Click here to learn more about our GMP manufacturing capabilities and the path to regulated manufacturing.
Contact Us
We are excited to learn more about your project. Contact us today to talk with our experts.



